Scales, spines, feathers and hair are examples of vertebrate skin appendages, which constitute a remarkably diverse group of micro-organs. Despite their natural multitude of forms, these appendages share early developmental processes at the embryonic stage. We have discovered how to permanently transform the scales that normally cover the ventral footpads of chickens into feathers, by specifically modifying the expression of certain genes. These results, published today in the journal Science Advances, open new perspectives for studying mechanisms that have enabled radical evolutionary transitions in form among species.
The skin of terrestrial vertebrates is adorned with diverse keratinized appendages, such as hair, feathers, and scales. Despite the diversity of forms within and among species, the embryonic development of skin appendages typically begins in a very similar way. Indeed, all of these structures develop from cells that produce a localized thickening on the skin surface and express particular genes. One of these genes, Sonic hedgehog (Shh), controls a signaling pathway involved in the development of diverse structures, including the neural tube, limb buds and skin appendages.
A common ancestor
Our laboratory is interested, among others, in the physical and biological processes that generate the diversity of skin appendages in vertebrates. In particular, our group has previously demonstrated that hair, feathers and scales are homologous structures inherited from a reptilian common ancestor.
Feathers of the chicken embryo are used as a model system to understand skin appendage development. While it is known that certain breeds of chickens, such as the ‘Brahma’ and ‘Sablepoot’ varieties, exhibit feathered legs and dorsal foot surfaces, the genetic determinism of this trait is not fully understood.
A transient modification for a permanent change
As the signaling pathways responsible for this transformation have not been fully determined, we investigated the potential role of the Shh Pathway. Rory Cooper and Michel Milinkovitch used a technique of ‘egg candling’, in which a powerful torch illuminates blood vessels on the inside of the eggshell. This allowed them to precisely treat chicken embryos with a molecule that specifically activates the Shh pathway, injected directly into the bloodstream.
We observed that this single stage-specific treatment is sufficient to trigger the formation of abundant juvenile down-type feathers, in areas that would normally be covered with scales. Remarkably, these experimentally-induced feathers are comparable to those covering the rest of the body, as they are regenerative and are subsequently and autonomously replaced by adult feathers.
After comparison with embryos injected with a control solution, RNA sequencing analysis showed that the Shh pathway is both immediately and persistently activated following injection of the molecule. This confirms that activation of the Shh pathway underlies the conversion of scales into feathers.
Our results indicate that a developmental leap – from scales to feathers – does not require large changes in genome composition or expression. Instead, a transient change in expression of one gene, Shh, can produce a cascade of developmental events leading to the formation of feathers instead of scales. This research, initially focused on the study of the development of scales and feathers, therefore has important implications for understanding the evolutionary mechanisms generating the enormous diversity of animal forms observed in nature.
Much additional details are available in the original publication:
Transient agonism of the sonic hedgehog pathway triggers a permanent transition of skin appendage fate in the chicken embryo
Cooper R. L. & M. C. Milinkovitch
Science Advances 9, eadg9619
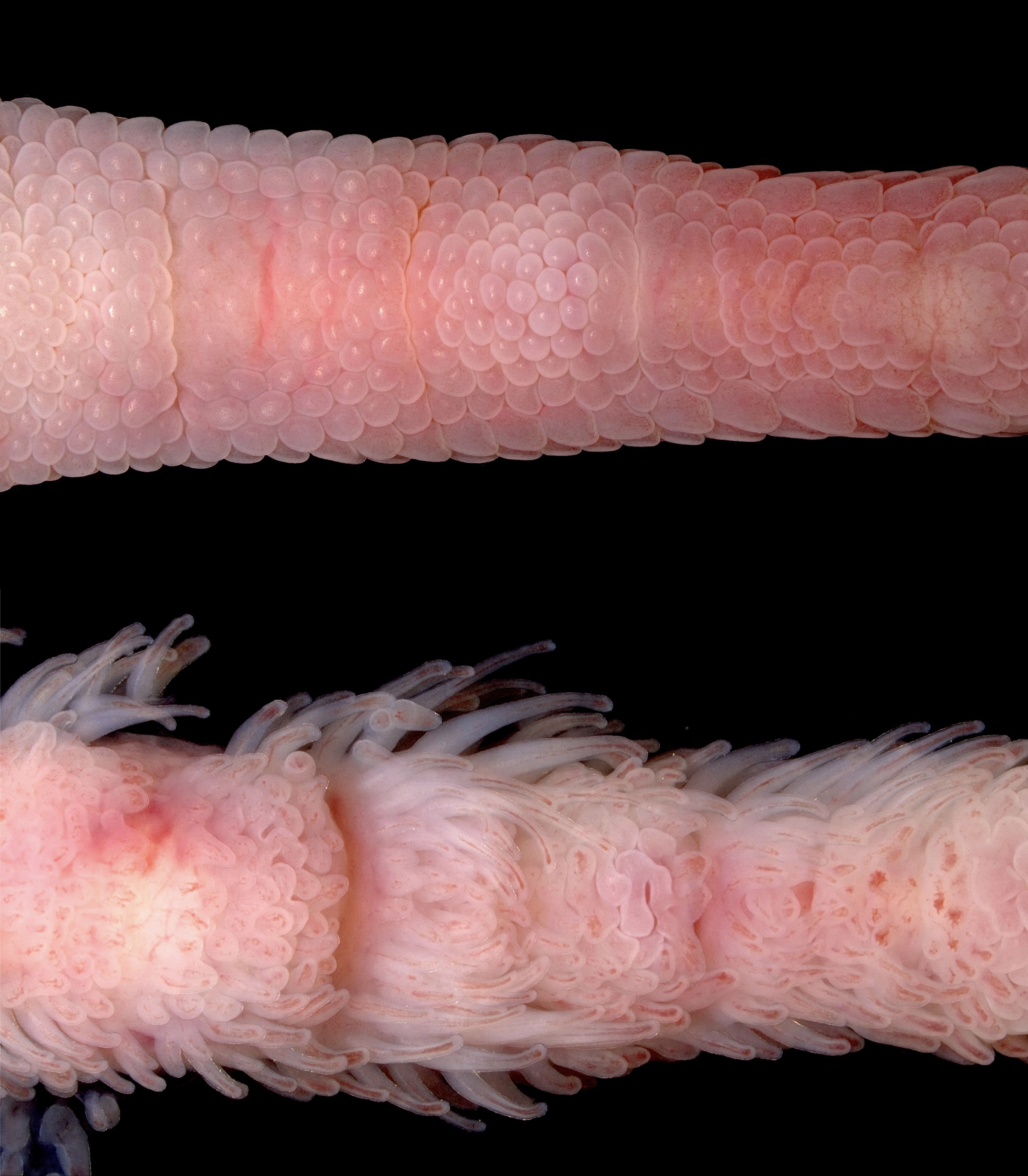
Image 1: Agonism of Shh pathway signalling alters skin appendage fate and regionalisation. Samples at embryonic day 11 were treated with either DMSO (controls) or 200 µg SAG. Control sample (top) fixed 5 days post treatment (embryonic day 16) reveal normal patterning of reticulate scales on the ventral digit surface, whereas SAG treatment (bottom) triggers abundant ectopic feather bud development on both ventral and lateral digit surfaces, in regions that would normally form reticulate scales and scutella.
Video 1: SAG-induced ectopic feather buds on the avian footpad.Rotating animation showing a 3D reconstruction of light-sheet fluorescence microscopy imaging of a SAG-treated embryonic chicken digit sample fixed at embryonic day 16 (i.e., 5 days post SAG treatment).